Toddler becomes first child in UK to receive revolutionary gene therapy
A 19-month-old girl has become the first child in the UK to receive a revolutionary gene therapy for a fatal disorder.
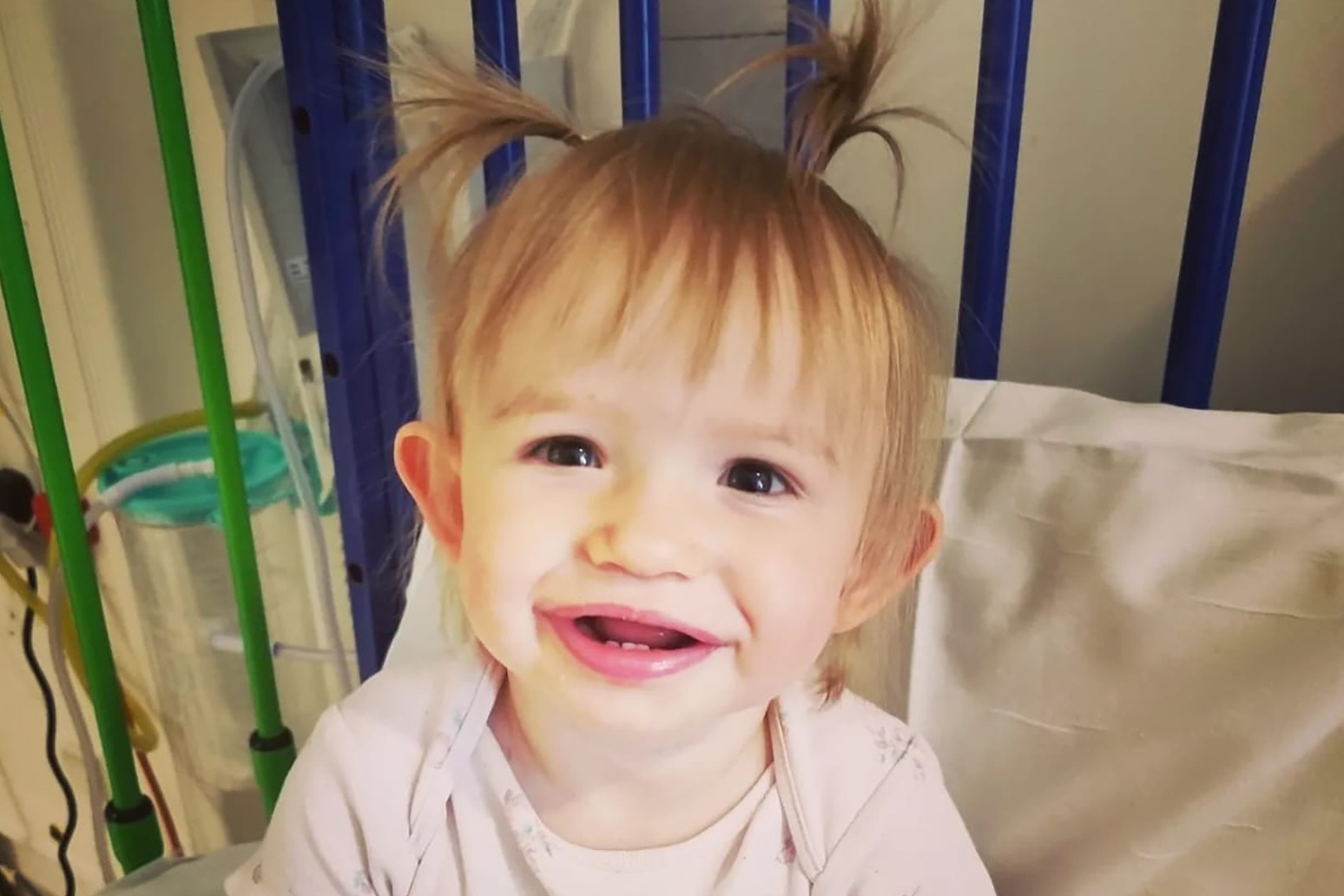
Teddi Shaw suffers from deadly metachromatic leukodystrophy (MLD) but has been given the chance of a normal life thanks to one of the world’s most expensive drugs.
The therapy, Libmeldy, has been approved on the NHS for the condition, which is inherited and rare and causes serious damage to the nervous system and organs, dramatically cutting life expectancy.
The treatment had a list price of £2.8 million when it was approved last year, but NHS England reached agreement with the firm Orchard Therapeutics to offer it at a discounted price.
Libmeldy is a treatment that works to correct the genetic cause of MLD by inserting functional copies of a faulty gene into the patient’s own stem cells.
The stem cells come from the patient’s own bone marrow or blood and are then fed back into the body carrying the new genetic information.
Teddi, from Northumberland, had her stem cells removed and the faulty genes replaced in several stages between June and October last year.
She is now a healthy and happy toddler and shows no signs of the devastating disease she was born with.
However, her family are still facing heartbreak because her three-year-old sister, Nala, who also suffers MLD, is too far advanced in her illness to benefit from the treatment.
The therapy, which is delivered as a one-off intravenous infusion, must be given before the irreversible damage caused by the disease progresses too far, according to guidance from the National Institute for Health and Care Excellence (Nice).
Teddi and Nala live with their parents Ally, 32, and Jake, 29.
Mrs Shaw said: “In April last year, our world was turned upside down when not one but both of our daughters were diagnosed with MLD.
“Being told our first daughter, Nala, wasn’t eligible for any treatment, would continue to lose all functions and die extremely young was the most heartbreaking and hardest thing to come to terms with.
“However, amongst the pain, was hope for our younger daughter, Teddi. We were told that a new gene therapy treatment had, luckily, recently been made available on the NHS.
“We are extremely privileged that Teddi is the first child to receive this on the NHS and grateful that she has the opportunity to lead a long and hopefully normal life. Without this treatment, we would be facing both our children being taken away.
“We can only hope that, one day, a treatment becomes available for all stages of MLD, and we feel strongly that it should be added to the newborn screening test to save more families from having to go through this heartache.”
She added: “Teddi is doing absolutely brilliant. She is walking, running, a chatterbox – absolutely no signs so far of MLD. She is an absolute character and has everyone around her laughing all the time.”
Only around five children are born each year in England with MLD.
According to Nice, the condition is caused by a lack of the enzyme Arylsulfatase-A.
Without this enzyme, substances called sulphatides build up, eventually destroying the protective myelin sheath of the nervous system.
As a result, the nerves in the brain and the peripheral nerves cease to function properly, causing symptoms including muscle weakness, sight and hearing loss, difficulty walking, loss of speech, cognitive decline and seizures.
Children whose MLD starts before 30 months (the most common and the most rapidly progressing type) deteriorate quickly and usually die between the ages of five and eight.
Those whose MLD starts between 30 months and six years of age have a life expectancy of 10 to 20 years more.
Libmeldy is being made available on the NHS as a specialist service through the Royal Manchester Children’s Hospital.
This centre is one of just five European sites administering the treatment, and is the only site in the UK.
NHS chief executive Amanda Pritchard said: “This is a huge moment of hope for parents and their babies who are born with this devastating inherited disorder, that can now be treated with a single round of revolutionary treatment at a specialist centre on the NHS.
“I am delighted that we have given this miracle treatment to the Shaw family at what must have been a horrendous time for them, and I would like to thank the staff at Royal Manchester Children Hospital for turning research into reality for Teddi and others who will benefit.”
Professor Rob Wynn, director of the paediatric bone marrow transplant programme at the Royal Manchester, said: “Being able to offer this first licensed treatment as part of NHS standard of care and, crucially, transform Teddi’s life, has been an exciting experience for all of us involved here in Manchester – staff, researchers, patients and families.
“Through the years, colleagues and I have looked after a range of patients with rare but severe conditions, where treatment has been limited.
“It is wonderful to be involved in this breakthrough moment and deliver a gene therapy which will transform outcomes for patients with MLD.”
Professor Simon Jones, a clinical director at the Royal Manchester, said: “MLD is a progressive, life-limiting condition and, prior to this metabolic disorder service being made available via the NHS, there were no approved treatment options available.”
Vivienne Clark, chairwoman of MLD Support Association UK, said she is “thrilled” that Teddi is now expected to lead a normal life.
Health Secretary Steve Barclay said: “Gene therapy is transforming healthcare and, most importantly, saving lives.
“Thanks to treatments such as Libmeldy, children like Teddi and their families can avoid heartbreak and spend more precious moments together.”
Published: by Radio NewsHub